APEX2-based Protein Proximal Labeling
APEX2 Proximal Labeling Protocol
Advantage
- Capture weak or transient interactive proteins.
- Detect proteome at “inaccessible” regions of interest which can not be purified, such as mitochondrial intermembrane space (IMS).
Plasmid
APEX2-V5-POI
POI: proteins of interest
Buffer
500mM Biotin-phenol母液
称取0.05g Biotin-phenol,溶于278μL DSMO,分装(每管30μL)后-20℃储存.
500μM Biotin-phenol in 新鲜培养基
1:1000在新鲜培养基中加入500mM Biotin-phenol母液.5mM H2O2 in 新鲜培养基(第3步快结束时现配)
5.11μL*30%过氧化氢,加入10 mL新鲜培养基中.反应终止液(PBS with 10mM sodium ascorbate, 5mM TROLOX, 10mM sodium azide)
先配置储液0.198g sodium ascorbate in 1mL water (100X), 0.125g TROLOX to 1mL DMSO.注意这两个储液也不能真的去“储”,使用时现配!
使用时按1:100加入100mL PBS中.
RIPA buffer (现加cocktail和PMSF)
50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 使用时现加cocktail和PMSF)有的Protocol推荐:RIPA lysis buffer supplemented with 1× protease inhibitor cocktail, 1 mM PMSF and quenchers (10 mM sodium azide, 10 mM sodium ascorbate and 5 mM Trolox)
1M KCl溶液
0.1M Na2CO3溶液
2M尿素 in 10mM Tris-HCl, pH8.0
洗脱液(3x protein loading buffer supplemented with 20mM DTT and 2mM biotin)
Procedure
Biotin labeling
细胞传至2个10cm板长满
弃培养基,每个10cm板加入4mL (500μM Biotin-phenol in 新鲜培养基)
放入37℃ CO2培养箱静置30min
现配1mL (5mM H2O2 in 新鲜培养基),立刻从培养箱中取出细胞,迅速将1mL (5mM H2O2 in 新鲜培养基)加入10cm板中(H2O2终浓度1mM),在手中温柔摇晃孵育1min.
不要同时操作超过2盘细胞。
迅速弃上清,迅速加入4mL反应终止液(PBS with 10mM sodium ascorbate, 5mM TROLOX).
温柔摇晃5s,弃上清,加入4mL反应终止液.
温柔摇晃5s,弃上清,加入4mL反应终止液.
温柔摇晃5s,弃上清,加入2mL预冷的PBS.
吹打细胞,收集至15mL离心管(两个板2mL+2mL).
3000 x g, 4℃, 离心5cm,弃上清,细胞pellet冻存-80℃.
Streptavidin beads enrichment of biotinylated proteins
- 冰上解冻细胞.
- 加入1.5mL RIPA buffer,吹打混匀,4℃慢速旋转裂解5min.
- 15,000 x g, 4℃离心10min,收集上清液,测蛋白浓度.
- 取450 μL Streptavidin-coated magnetic beads (Pierce),RIPA Buffer洗两次.
- 弃上清,向beads沉淀中加入8mg蛋白溶液,室温慢速旋转孵育1h或4℃慢速旋转孵育过夜.
- 1mL RIPA buffer洗两次.
- 1mL 1M KCl溶液洗一次.
- 1mL 0.1M Na2CO3溶液洗一次.
- 1mL (2M尿素 in 10mM Tris-HCl, pH8.0)洗一次.
- 1mL RIPA Buffer洗两次.
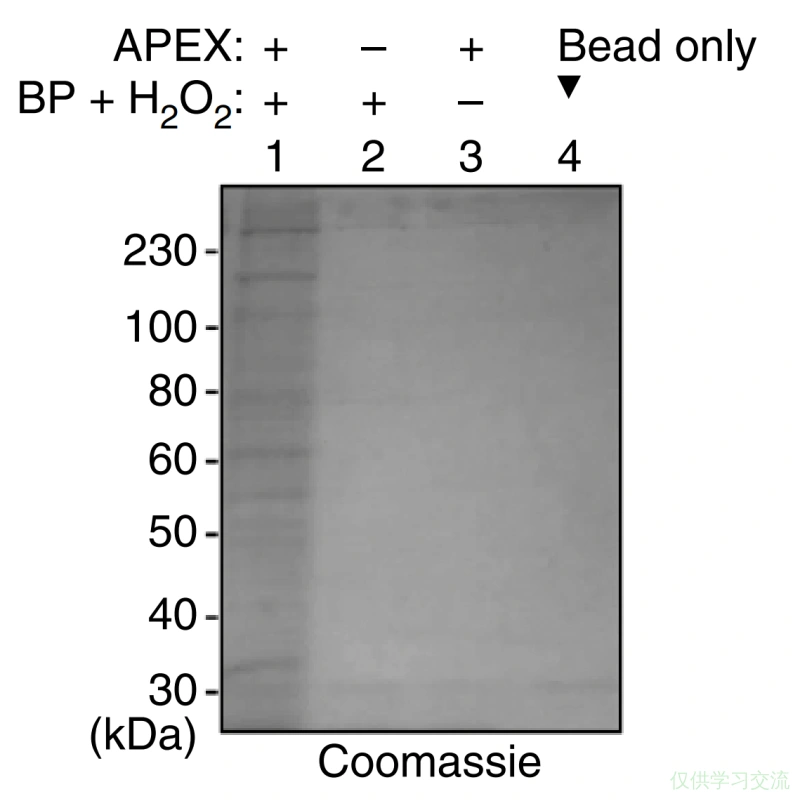
- 加入75μL (3x protein loading buffer supplemented with 20mM DTT and 2mM biotin),跑SDS-PAGE和Strep-WB.
- 质谱分析.
Optimization
Control 1 — Negative control [no-H2O2 or no-BP (biotin-phenol)]
Control 2 — Mass spectrometry control
根据具体的目标蛋白定位来选择,单纯的no-APEX2 or no-H2O2 Control或者别的蛋白construct的Control
Imaging APEX2-V5-POI localization
Optimize Streptavidin pull-down
原文推荐:360 µg (~90 µl) of each whole-cell lysate sample with 30 µl of streptavidin magnetic beads (Pierce).
可以使用小样(6-well plate的一孔细胞,大概2.5Million)进行条件摸索。
Critical points
- 不能使用BCA法对蛋白进行定量,Quench buffer中的成分对BCA法有干扰;
- 不要在fusion construct中使用HA标签,HA标签可能因为被标记biotin而失效;
- 不要同时操作2盘以上细胞,以免影响H2O2标记时间,无法及时终止;
- 抗坏血酸钠和TROLOX一定要从干粉现配现用,不能存;
- H2O2要在开始使用前几分钟现配,以免分解;